Purging means the replacement of an atmosphere of undesired composition in an enclosure by another atmosphere of required composition. MADEL OIL AND GAS LIMITED has used Nitrogen extensively for replacing an active and explosive atmosphere because it is odorless tasteless, non-toxic and practically inert. The objective is to reduce the hydrocarbon level to below its Lower Explosive Level (LEL) so that on contact with air, the hydrocarbon concentration is insufficient to create an explosive mixture with atmospheric oxygen.
Nitrogen purging is used for both start up and shutdown operations in order to replace the existing system atmosphere with safe and inert nitrogen blanket. During start up operations, purging is performed to reduce the oxygen content to typically below 2% by volume and thus prevent any explosive mixtures being formed when hydrocarbons are introduced. During shutdown operations, the hydrocarbon content is purged out of the system to leave an inert atmosphere, which is below the lower explosive limit of that hydrocarbon.
Our subsequent experience on similar projects, we reckoned that both the designated vessels and their corresponding piping should be purged. Secondly, due to no information as per the piping isometrics etc, we intend to perform both displacement and dilution purging in order to reduce the hydrocarbon level to acceptable Lowest Explosive Limit
(LEL).
Displacement Purging
In this method, Nitrogen is pumped continuously into a vessel or system at one point, whilst the air or gas leaves from another point. The purged gas is normally vented to atmosphere so that the system remains at atmospheric pressure during the entire purging operation. The purging operation continues until the hydrocarbon content of the piping is less than 4% by volume, and this will be determined by the use of a gas meter which will be provided by MOG and or CLIENT.
The Nitrogen performs its function by displacement and / or dilution of the purged gas. In the typical case, both of these processes occur simultaneously. In a long narrow vessel such as a pipe, Nitrogen is passed in at one end and the purged gas leaves the other. The purge may take place almost entirely by displacement, with little mixing of Nitrogen and purged gas at the relatively small interface. In such cases, the amount of Nitrogen required to remove virtually all of the purged gas is almost the same as the volume of the pipe.
When performing a displacement purge, the pump rate should be moderate. If the pump rate is too slow it will allow time for diffusion and tends to result in a purge mainly by dilution. Similarly, a high flow rate and short purging time gives a turbulent jet of Nitrogen at the injection point which also tends towards mixing and purging by dilution. An intermediate flow rate reduces the chance of this happening, increases the amount of purge by displacement and uses the nitrogen more efficiently. As each purging application has a different set of circumstances, it is not possible to calculate optimum flow rates. Gas analysis and previous experience with similar systems can only determine this.
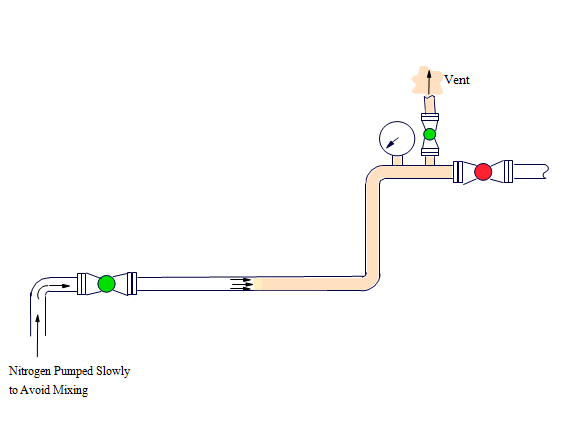
As mentioned previously, this method is unsuitable for systems with complex piping work or with obstructions such as baffles or pipe coils, etc. In such cases, short-circuiting of the nitrogen between the injection and vent points may occur, leaving hydrocarbons trapped in “dead legs.” In instances such as these, an alternative method should be used such as dilution purging.
Dilution Purging
In this method, the equipment or vessel to be purged is pressurized with Nitrogen and left for a period to allow for complete mixing of Nitrogen and initial equipment atmosphere. The pressure is then released, generally to atmosphere. This method is restricted to equipment which can withstand purge design pressure.
It is also advantageous in cases where pressurization is necessary for some other purpose, such as leak testing for example or when only one connection is available for inlet and outlet.
In this method the amount of Nitrogen required to reduce any gas concentration from a known initial level to a desired final level is exactly calculable.
In the typical case, the required purge can be obtained by one or several successive cycles of pressurising and venting. The number of cycles depends on the pressure which the system can withstand, the initial gas concentration, the final gas concentration required and any gassing off which occurs during the purging operation.
The Nitrogen performs its function by displacement and / or dilution of the purged gas. In the typical case, both of these processes occur simultaneously. In a long narrow vessel such as a pipe, Nitrogen is passed in at one end and the purged gas leaves the other. The purge may take place almost entirely by displacement, with little mixing of Nitrogen and purged gas at the relatively small interface. In such cases, the amount of Nitrogen required to remove virtually all of the purged gas is the same as the volume of the pipe.
In more general cases, such as purging of separators, total displacement purging is almost impossible due to the restrictions of flow within separators caused by obstructions such as baffles. In this case purging is carried out almost exclusively by dilution.
Purging to a given concentration by dilution as opposed to displacement obviously uses more Nitrogen. When calculating the amount of Nitrogen required, it is best to assume that complete mixing will occur, as this will give a conservative estimate of Nitrogen requirements.
Injection and Venting Points
When selecting injection or venting points, the type of purge, the system to be purged and the composition of the undesired atmosphere must be taken into account.
When performing a displacement purge, injection and vent points should be at opposite ends of a system, away from any obstructions that may increase mixing.
When performing a dilution purge, the location of injection point is not critical. When venting, numerous points should be used to ensure “dead legs” etc are purged through.
When system vents are not in operation extreme care should be taken when venting. Gas should never be vented into enclosed modules. If gas denser than air is vented, extreme care should be taken to ensure that dense gasses do not collect in low areas to form explosive mixtures. In all cases when venting, it must be ensured that no gas is trapped due to non-return valves.
Gas Analysis
It is important to remember when taking gas samples from a system, that various sample points are used. Opposite ends of a system, as well as possible “dead leg” areas should be sampled.
Ensure that all meters used have up to date calibration certificates and are functioning properly before using. The two main gas meters used by MOG are the oxygen meter for measuring oxygen content and a hydrocarbon in Nitrogen meter, which is calibrated to measure LEL.